By Monica Esopi
Overview:
This lesson is intended to provide an introduction into some key concepts within the field of chemical engineering through hands-on demonstrations and activities. The concepts covered include heat transfer, kinetics, mass transport, and separations. These can be related to everyday life and give students an idea of what the concepts mean and how they can relate to chemical engineering, ideally providing some insight into what the field encompasses.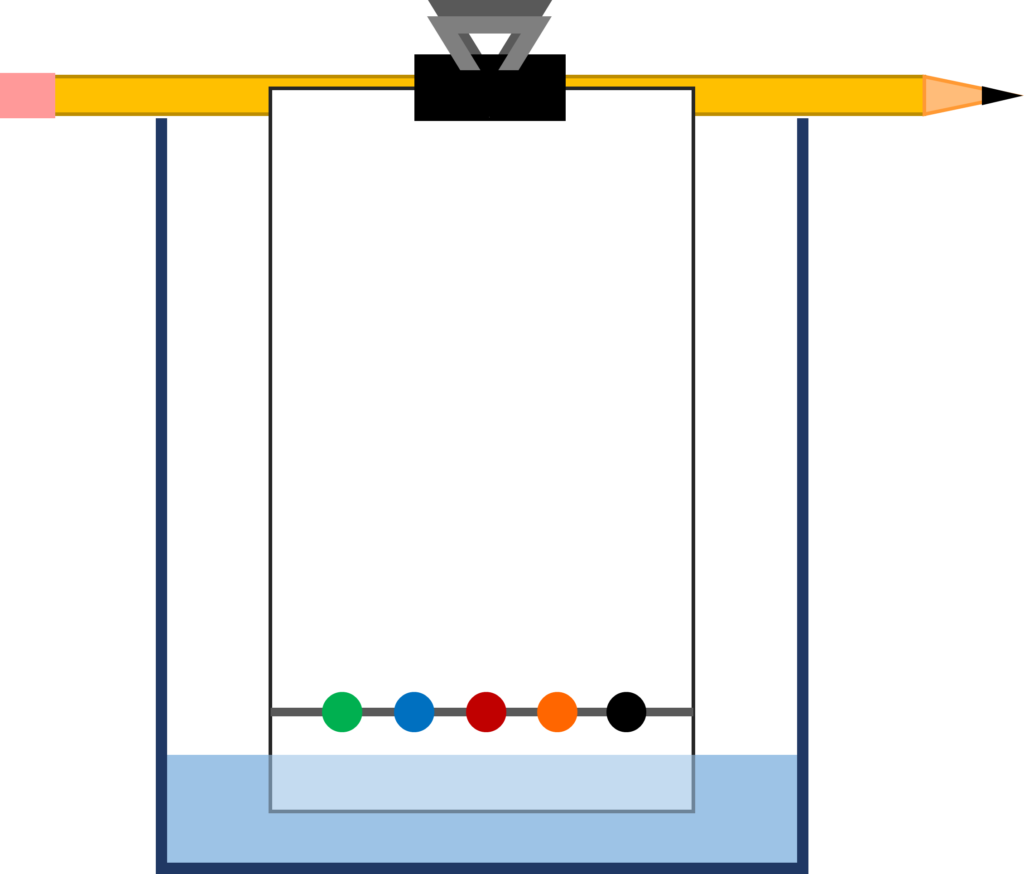
Essential Question:
What is Chemical Engineering?
Background:
Chemical engineering is the application of fundamental principles of math, chemistry, physics, and economics to improve the safety, quality, and efficiency of chemical processes. These chemical processes could be in a variety of fields including petrochemicals, commodity and specialty chemicals, food production, paints and dyes, consumer products, and many more. Chemical engineering also incorporates nanoscience and aspects of electronics and biology. It is an incredibly broad field that is constantly growing and expanding, making it difficult for those outside the field, especially young students, to have a clear idea of what the field is.
Three core concepts in chemical engineering are heat transfer, reaction kinetics, and mass transport.
Heat transfer is an essential component of chemical processing and can occur through three main mechanisms: conduction, convection, and radiation. The heat transfer demo/activity in this lesson focuses on conduction. An ice cube will be put into contact with two different materials, metal and plastic, to see which will cause the ice to melt faster. Because metal conducts heat, and plastic does not, when an ice cube is in contact with a metal block, heat can transfer from the metal into the ice cube very quickly, causing the ice cube to melt much faster than the plastic block does.
Kinetics deals with the speed of chemical reactions. When dealing with and controlling chemical reactions, the rate of those reactions is critical to understand and predict. In this demo/activity, we can visually demonstrate the impact of temperature on reaction kinetics using glow sticks. Glow sticks work because of a chemical reaction that produces light. Generally, increasing the temperature of a reaction increases its speed due to an increase in the energy of the system. The molecules have greater kinetic energy and collide more frequently, making the overall reaction faster. Heating glow sticks increases the rate of the reaction, causing it to glow more brightly right away, but there is only a certain amount of the chemical in the glow stick, so it is used up more quickly and therefore fades quickly. Cooling the glow sticks makes them burn less brightly, but they will last longer since they use the chemicals more slowly.
Mass transport refers to how material moves (e.g. wind, water currents, liquids flowing through pipes, snow falling down a mountain, etc)., and separations deals with the separation of different materials. There are many different ways to accomplish this including filters, membranes, distillation, extraction, etc. Separations is a huge part of chemical engineering and one example, which relies on mass transport phenomena, is chromatography. In this activity, chromatography can be done with household items to separate the different color inks in non-primary-color markers. It relies on capillary action, which refers to liquid flowing in very narrow spaces or along a material, sometimes in the “wrong direction”. A great, relatable example is how the water creeps up your legs if you wear pants that touch the ground when the ground is wet. In this activity, As the water moves up the paper, it takes the dyes with it. Because of the chemical structures and sizes of the dyes, some will move with the water more easily than others, so they will move at different speeds. For example, if there are both blue and red dyes in a purple marker, the blue dye may be a smaller molecule than the red dye and therefore move more easily, so as the water moves up the paper the blue dye will travel farther than the red and the two will be physically separated and you might end up with a blue spot above a red spot.
Research Connection:
Chemical engineers do work and conduct research in a huge variety of areas, including many that relate to clean energy like materials, manufacturing, nanotechnology, electronic devices, electrochemistry, and more. The thing that makes the field difficult to understand is its breadth, which also makes it important and worth spreading awareness around.
.
NGSS Standards:
| Standard Number | Standard text |
| MS-PS3-4 | Plan an investigation to determine the relationships among the energy transferred, the type of matter, the mass, and the change in the average kinetic energy of the particles as measured by the temperature of the sample. |
| MS-PS1-2 | Analyze and interpret data on the properties of substances before and after the substances interact to determine if a chemical reaction has occurred. |
Materials:
Heat Transfer Activity (Melting Ice Cubes)
- Block of metal
- Block of plastic
- Ice (cooler for transport)
Kinetics Activity (Glow Stick Control)
- Glow sticks (at least two)
- 2 large beakers half-filled with water
- Hot plate
- Ice (cooler for transport, can be same as for heat transfer activity)
Mass Transport/Separations Activity (Separating Colors in Markers)
- Beaker
- Clamp
- Strip of filter paper (e.g. coffee filters cut into strips)
- Pencil
- Felt-tip markers
- Water (~0.1% NaCl is best but will work with plain water)
Procedure:
Heat Transfer Activity (Melting Ice Cubes)
- Hold both the metal and plastic blocks and make a prediction for which material might melt an ice cube faster.
- Place an ice cube on each block. Observe and record any differences between the two materials. Does the result match your prediction?
Kinetics Activity (Glow Stick Control)
- Make a warm water bath in the first beaker: place it on the hot plate and let the water warm up – above room temperature but not boiling
- Make an ice bath in the second beaker
There are two options for the next steps, you can do both if you have enough glow sticks
Option 1:
- Place one glow stick in each water bath and allow them to acclimate to the temperatures of the baths
- Take the glow sticks out of the baths and crack them, observe and record any differences
Option 2:
- Crack two glow sticks
- Place one in each water bath, observe and record any differences as they acclimate to the temperatures of the baths
Mass Transport/Separations Activity (Separating Colors in Markers)
- With the pencil, draw a straight line horizontally across the filter paper, approximately an inch from the bottom of the paper
- Make dots with the markers across the line. Choose as many colors as you’d like, but make sure the dots are as small and light as possible and keep approximately a ½ inch of space between them. What colors might be in each marker color you’ve chosen?
- Fill the beaker with approximately ½ inch of water
- Lay the pencil across the top of the beaker and clamp the filter paper to it so that the paper is standing vertically with its bottom in the water. It is important that the water level is below the line you drew. The final setup should resemble the included diagram.
Extensions:
Heat Transfer Activity (Melting Ice Cubes)
Other applications of insulators/conductors further demonstrate this point include: metal pans with plastic handles, which don’t conduct heat, so you don’t burn yourself; metal ice cream scoops, which helps the ice cream melt and makes it easier to scoop; plastic ice cube trays, so they don’t melt as quickly. The concept of conduction also applies to electricity, metal conducts and plastic doesn’t so metal wires are wrapped in plastic cables.
Kinetics Activity (Glow Stick Control)
Putting a glow stick in the freezer will make it last for a long time. Cooking food often involves heating and chemical reactions; hotter temperatures cook food faster and eating a solution helps it to dissolve (tea/coffee, jell-o).
Mass Transport/Separations Activity (Separating Colors in Markers)
If a black marker hasn’t been tested, this is a good one to include – it should separate out into several different colors. Can then discuss how this concept, the difference in the way that materials move and flow, can be used to analyze and separate materials.
Resources:
What is Chemical Engineering Lesson
This lesson plan was adapted from a workshop developed by the student organization Women in Chemical Engineering at the University of Washington.
The following write-up for the chromatography activity may be useful: https://www.scientificamerican.com/article/chromatography-be-a-color-detective/



