Electrochemical Chameleon
By Katie Corp – Schlenker Research Group
Students experiment with acidity of solutions and then use electricity to split water into hydrogen and oxygen and observe changes in the solution.
QUESTION
How can water be split into its simple elements?
Background
One way to produce hydrogen gas and oxygen gas for energy storage is water splitting via electrolysis. Electrolysis is a process of using an electrical current to drive a chemical reaction that would otherwise not happen, or is non-spontaneous. In this lab, we will electrolyze normal tap water (H2O) using a common 9-volt battery as the energy source and Epsom salt as the electrolyte to facilitate the reaction. A natural anthocyanin pH indicator extracted from red cabbage is added to the water before splitting to show the changes in pH values in the solution during electrolysis via vivid color changes. During the experiment, oxygen gas (O2) is generated at the thumbtack that connects to the positive terminal of the battery. Additionally, hydrogen ions (H+) are produced at the same location. With excessive hydrogen ions present, the solution at the positive terminal turns acidic and red.
2?2?→?2+4?+ + 4?−(oxidation)
On the other hand, at the thumbtack connected to the negative terminal of the battery, water is reduced to hydrogen gas and hydroxide (OH–), which makes the solution basic, causing the color to change to green.
2?2?+2?−→?2+2??−(reduction)
Note that the products of water splitting are hydrogen gas (H2) and oxygen gas (O2), which are both electrically neutral species, or molecules or compounds with no net charge. Hydrogen ions and hydroxides are only byproducts produced in the redox process.
In order to limit the negative impacts of climate change, decarbonization (elimination of greenhouse gas emitting processes) of the electric grid (the network that transports electricity from power generation facilities to consumers) is necessary. Clean energy technologies (systems that generate electricity from sources that are constantly replaced and do not generate greenhouse gasses) provide a solution to this issue. However, most clean energy technologies are intermittent, meaning they only produce electricity at certain times. One way to overcome this challenge is to store the extra electrical energy from clean energy sources as chemical energy, or energy that is stored by making or breaking chemical bonds. Common methods of doing this are by using large batteries or producing hydrogen and oxygen gas from water in a process known as electrolysis. These gasses can be burned, or used in a hydrogen fuel cell, a device that uses the reaction between hydrogen and oxygen gas to produce electricity, to recover the stored energy.
In this lab, we will use pH measurements to understand the reactions occurring during water splitting.
One way to track the progress of a chemical reaction is by monitoring the pH (the acidity or basicity) of the reaction solution. In this lab, you will begin by creating a range of pH reference solutions, and then use these solutions to understand the results of your experiment with storing electrical energy as chemical energy by splitting water into its component parts, hydrogen and oxygen.
Research Connection
Researchers are developing new catalysts for water splitting. Some can split water directly when exposed to sunlight– photocatalysis.
NGSS Standards
| Standard Number | Standard text |
| 4-PS3-4 | Apply scientific ideas to design, test, and refine a device that converts energy from one form to another.* |
| MS-PS1-2 | Analyze and interpret data on the properties of substances before and after the substances interact to determine if a chemical reaction has occurred. |
| MS-PS1-5 | Develop and use a model to describe how the total number of atoms does not change in a chemical reaction and thus mass is conserved. |
| HS-PS3-3. | Design, build, and refine a device that works within given constraints to convert one form of energy into another form of energy.* |
Materials
- Vinegar
- Lemon juice
- Sprite
- Tap water
- Antacid
- Baking soda
- Baking powder
- Ammonia
- 9V battery
- Thumbtack
- Soft Plastic cup like tupperware
- Plastic test tubes
- Red cabbage indicator – add 4-5 scoops of the powdered cabbage juice for each liter of water used. For a classroom with 12 pairs, 2-3 liters should suffice.
- Solar cell with soldered wires (extension)
- Copper tape (extension)
Procedure
Hazards:
Vinegar (an acid) and ammonia (a base) can irritate the skin and eyes. Avoid direct contact with skin and eyes. In the event of direct contact, flush with water for 15 minutes. Natural anthocyanin dyes can stain clothing and skin. Avoid spilling anthocyanin dyes on clothing and skin. H2 and O2 gasses are flammable. Keep the evolved gasses away from sparks and fire.
Warm Up:
- Write the following reaction on the board: 2C8H18 + 25O2 → 18H2O + 16CO2
- Ask students if they recognize the reaction. Inform them that it is the combustion of octane, a key component of gasoline. Ask them if they can identify the problem with using fossil fuels based on the reaction.
- Explain that many countries are replacing fossil fuels with clean energy technologies. Solar energy is one example. Ask them if they can think of challenges with using solar energy (hopefully someone will mention that the sun doesn’t always shine).
- Discuss energy storage as a means of overcoming this issue. During times when electricity is produced in excess of consumer demand, the electrical energy can be stored as chemical energy for later use.
- One example is in batteries. The second, which is relevant to this lab, is hydrogen from water splitting, or electrolysis. This hydrogen can be burned to produce thermal and mechanical energy, or alternatively, used in a fuel cell to generate electricity Ask students to predict the formula for this reaction. Write it on the board (remember to erase it before the lab, they will be asked to write it later). 2H2 + O2 → 2H2O
- Ask students what the benefit of using hydrogen as a fuel is, compared to octane.
- Explain that hydrogen can also be used to generate electricity by reacting it with oxygen in a hydrogen fuel cell.
Procedure:
- Explain that today, students will be experimenting with water splitting. They will use pH (-log([H+]) to track the reactions occurring. They will use anthocyanin dye from red cabbage juice as a pH indicator, or a chemical that changes color depending on the pH. First, they will create reference solutions to calibrate the color of the indicator ranging from basic to acidic. Calibration is an important technique where we measure a sample with a known quantity to obtain a signal (in this case color). We use a range of known samples to understand how the value of the quantity relates to the signal. This relationship gives us a calibration curve, which allows us to convert the signal from an unknown sample into the quantity of interest.
- After this, they will use an electrical energy source to split water, and interpret their observations using their reference solutions.
Protocol for Acid Bath pH indicator lab:
- Pair up with a partner and obtain eight vials from the stock station for your group
- Number your vials 1 – 8 with a permanent marker
- Halfway fill each of your vials with the stock pH indicator solution
- To vial 1 add a few drops of vinegar (an acid) and record your observations
- To vial 2 add a few drops of lemon juice and record your observations
- To vial 3 add a few drops of Sprite and record your observations
- To vial 4 add a few drops of tap water and record your observations
- To vial 5 add a few granules of antacid and record your observations
- To vial 6 add a few granules of baking soda and record your observations
- To vial 7 add a few granules of baking powder and record your observations
- To vial 8 add a few drops of Ammonia (a base) and record your observations
- Protocol for Electrolysis Lab:
- Make two marks on the bottom of your plastic storage that are the same distance apart as the terminals of your 9-volt battery
- Insert one thumbtack into the bottom of the container at each of the marks that you have made as shown in Fig. 1 to form an electrolysis cell
- Pour purple pH indicator solution into your new electrolysis cell until the container is 3/4 of the way full
- Return to your work station and insert two plastic pipettes into the purple pH indicator solution
- Squeeze the bulb of each pipette one time and release it to fill each pipette with the same amount of solution
- Without removing the tips of the pipettes from the solution, place each pipette tip over one of the electrodes in your electrolysis cell
- With the help of your partner to stabilize the pipettes so that they don’t disconnect from the electrodes, place your electrolysis cell on top of your 9-volt battery so that each battery terminal is connected to only one of the thumbtacks that is inserted into the bottom of your cell.
- Record your observations here
- Remove the pipettes from the cell terminals and dispense their contents back into the electrolysis cell
- Add one teaspoon of Epsom Salt (magnesium sulfate, MgSO4) to your electrolysis cell and stir the resulting solution until it is almost entirely dissolved
- Insert the two plastic pipettes into the purple pH indicator solution again
- Squeeze the bulb of each pipette one time and release it to fill each pipette with the same amount of solution
- Without removing the tips of the pipettes from the solution, place each pipette tip back over one of the electrodes in your electrolysis cell again
- This time switch roles with your partner to stabilize the pipettes so that they don’t disconnect from the electrodes while your partner places the electrolysis cell on top of your 9-volt battery so that each battery terminal is connected to only one of the thumbtacks that is inserted into the bottom of your cell
- Record your observations
- Dispose of your solutions down the drain.
- Rinse out and return your glassware
Questions
- What happened at the positive battery terminal? (The solution turned red and gas evolved.)
- What happened at the negative battery terminal?( The solution turned green and gas (more) evolved.)
- How much solution was left in each of your pipettes when your experiment was finished? What does this mean about the volume of gas that was produced at each terminal? (Twice as much gas evolved on the negative terminal of the battery compared to the positive terminal.)
- From Question #3, explain how we know that water has the molecular formula H2O?( Twice as much hydrogen is available compared to oxygen in the formula for water (H2O), so more hydrogen gas is evolved in the water splitting process. )
- Do you think that the solution at the positive terminal was acidic, basic, or neutral? (The positive terminal of the battery will undergo oxidation, which means oxygen gas is formed which has a byproduct of hydrogen ions, lowering the pH so the solution turns red (see redox reaction below)
- Do you think that the solution at the negative terminal was acidic, basic, or neutral? (The negative terminal of the battery will undergo reduction, which means hydrogen gas is formed which has a byproduct of hydroxide ions, increasing the pH so the solution turns green (see redox reaction below)
Bonus
Bonus: Write the half reactions occurring at the electrodes (one at each electrode)
2H2O→2H2+O2 (full reaction)
2?2?→?2+4?+ + 4?−(oxidation)
2?2?+2?−→?2+2??−(reduction)
To get full reaction from the half reactions, the electrons must cancel. Thus, multiple the reduction half reaction by two and add the resulting equation to the oxidation half reaction.
Note that OH−+H+=H2O.
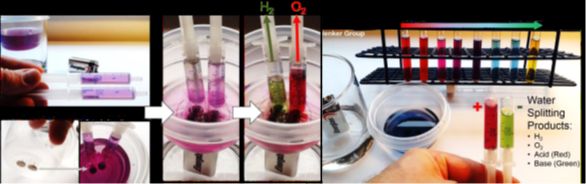
Fig. 1. We have extracted a natural anthocyanin dye from red cabbage. We use this dye as a universal pH indicator. The color change from purple to green or red allows us to show that splitting water into 2 parts H2 (larger volume of gas) and 1 part O2 (smaller volume) using a common 9-volt battery and two thumbtacks also generates acid (red) at the anode and base (green) at the cathode.
Extensions
- Try burning the hydrogen by bringing a match to the hydrogen tube. Blow out the match and while its still glowing place it in the oxygen filled tube.
- Demonstrate electrolysis / fuel cell with the fuel cell car listed below.
Resources
Where to get your materials
Electrochemical Chameleon Student Handout
(Printable version included on the lesson plan above)
One way to produce hydrogen gas and oxygen gas for energy storage is water splitting via electrolysis. Electrolysis is a process of using an electrical current to drive a chemical reaction that would otherwise not happen, or is non-spontaneous. In this lab, we will electrolyze normal tap water (H2O) using a common 9-volt battery as the energy source and Epsom salt as the electrolyte to facilitate the reaction. A natural anthocyanin pH indicator extracted from red cabbage is added to the water before splitting to show the changes in pH values in the solution during electrolysis via vivid color changes. During the experiment, oxygen gas (O2) is generated at the thumbtack (electrode) that is negatively charged, called the cathode. Additionally, hydrogen ions (H+) are produced at the same location. On the other hand, at the anode, or the negatively charged electrode, water is reduced to hydrogen gas and hydroxide (OH–). Note that the products of water splitting are hydrogen gas (H2) and oxygen gas (O2), which are both electrically neutral species, or molecules or compounds with no net charge. Hydrogen ions and hydroxides are only byproducts produced in the redox process.
In order to limit the negative impacts of climate change, decarbonization (elimination of greenhouse gas emitting processes) of the electric grid (the network that transports electricity from power generation facilities to consumers) is necessary. Clean energy technologies (systems that generate electricity from sources that are constantly replaced and do not generate greenhouse gasses) provide a solution to this issue. However, most clean energy technologies are intermittent, meaning they only produce electricity at certain times. One way to overcome this challenge is to store the extra electrical energy from clean energy sources as chemical energy, or energy that is stored by making or breaking chemical bonds. Common methods of doing this are by using large batteries or producing hydrogen and oxygen gas from water in a process known as electrolysis. These gasses can be burned, or used in a hydrogen fuel cell, a device that uses the reaction between hydrogen and oxygen gas to produce electricity, to recover the stored energy.
One way to track the progress of a chemical reaction is by monitoring the pH (the acidity or basicity) of the reaction solution. In this lab, we will use pH measurements to understand the reactions occurring during water splitting. You will begin by creating a range of pH reference solutions, and then use these solutions to understand the results of your experiment with storing electrical energy as chemical energy by splitting water into its component parts, hydrogen and oxygen.
Hazards
Vinegar (an acid) and ammonia (a base) can irritate the skin and eyes. Avoid direct contact with skin and eyes. In the event of direct contact flush with water for 15 minutes. Natural anthocyanin dyes can stain clothing and skin. Avoid spilling anthocyanin dyes on clothing and skin. H2 and O2 gases are flammable, avoid being in close proximity to sparks and fire.
Protocol for Acid Bath pH indicator lab:
- Pair up with a partner and obtain eight vials from the stock station for your group
- Number your vials 1 – 8 with a permanent marker
- Halfway fill each of your vials with the stock pH indicator solution
- To vial 1 add a few drops of vinegar (an acid) and record your observations
- To vial 2 add a few drops of lemon juice and record your observations
- To vial 3 add a few drops of Sprite and record your observations
- To vial 4 add a few drops of tap water and record your observations
- To vial 5 add a few granules of antacid and record your observations
- To vial 6 add a few granules of baking soda and record your observations
- To vial 7 add a few granules of baking powder and record your observations
- To vial 8 add a few drops of Ammonia (a base) and record your observations
Protocol for Electrolysis Lab:
- Make two marks on the bottom of your plastic storage that are the same distance apart as the terminals of your 9-volt battery
- Insert one thumbtack into the bottom of the container at each of the marks that you have made as shown in Fig. 1 to form an electrolysis cell
- Pour purple pH indicator solution into your new electrolysis cell until the container is 3/4 of the way full
- Return to your work station and insert two plastic pipettes into the purple pH indicator solution
- Squeeze the bulb of each pipette one time and release it to fill each pipette with the same amount of solution
- Without removing the tips of the pipettes from the solution, place each pipette tip over one of the electrodes in your electrolysis cell
- With the help of your partner to stabilize the pipettes so that they don’t disconnect from the electrodes, place your electrolysis cell on top of your 9-volt battery so that each battery terminal is connected to only one of the thumbtacks that is inserted into the bottom of your cell
- Do you observe any bubbles or color changes?
- Remove the pipettes from the cell terminals and dispense their contents back into the electrolysis cell
- Add one teaspoon of Epsom Salt (magnesium sulfate, MgSO4) to your electrolysis cell and stir the resulting solution until it is almost entirely dissolved
- Insert the two plastic pipettes into the purple pH indicator solution again
- Squeeze the bulb of each pipette one time and release it to fill each pipette with the same amount of solution
- Without removing the tips of the pipettes from the solution, place each pipette tip back over one of the electrodes in your electrolysis cell again.
- Switch roles with your partner to stabilize the pipettes so that they don’t disconnect from the electrodes while your partner places the electrolysis cell on top of your 9-volt battery so that each battery terminal is connected to only one of the thumbtacks that is inserted into the bottom of your cell
- Record your observations here
- Dispose of your solutions down the drain.
- Rinse out and return your glassware
Questions
- What happened at the positive battery terminal?
- What happened at the negative battery terminal?
- How much solution was left in each of your pipettes when your experiment was finished? What does this mean about the volume of gas that was produced at each terminal?
- From Question #3, explain how we know that water has the molecular formula H2O?
- Do you think that the solution at the positive terminal was acidic, basic, or neutral?
- Do you think that the solution at the negative terminal was acidic, basic, or neutral?
Bonus: Write the half reactions occurring at the electrodes (one at each electrode)



