Polymers
[vc_row][vc_column][mkd_section_title title="Polymers" title_size="large" title_color="" title_text_align="" margin_bottom="" width=""][vc_column_text]By Monica Esopi The intention of this lesson is to learn background about polymer materials and their applications, and to explore these materials through hands-on activities (making slime and bouncy balls). Students will be able to make their own polymers and explore their properties. These activities can be done individually, or in pairs or groups. Students will make slime to explore cross-linking, and then make bouncy balls to see the impact of a thickening agent.[/vc_column_text][vc_empty_space height="30px"][vc_hoverbox image="18213" primary_title="" primary_align="left" hover_title="QUESTION" shape="square" el_width="30" align="left"]How can simple molecules be joined together in chains or networks to make a substance with different...
Bubble Raft Crystal Model
[vc_row][vc_column][mkd_section_title title="Bubble Raft Crystal Model" title_size="large" title_color="" title_text_align="" margin_bottom="" width=""][vc_column_text]In this demonstration / lab students use uniform bubbles floating on water to model the formation and organization of atoms in crystals (Bubleraft Lesson)[/vc_column_text][vc_empty_space height="30px"][vc_hoverbox image="18209" primary_title="" primary_align="left" hover_title="QUESTION" shape="square" el_width="30" align="left"]Can we use bubbles floating on water to model the organization of atoms forming a crystal?[/vc_hoverbox][vc_empty_space height="40px"][mkd_accordion style="boxed_toggle" el_class="GLOWING COLORS"][mkd_accordion_tab icon_pack="" title="Background"][vc_column_text]Self-assembly is the idea that particles can organize themselves into the complex structures with a high amount of order. Closely packed balls of the same size will eventually sort themselves into tightly packed rows that look like the atoms arranged in crystals. At...
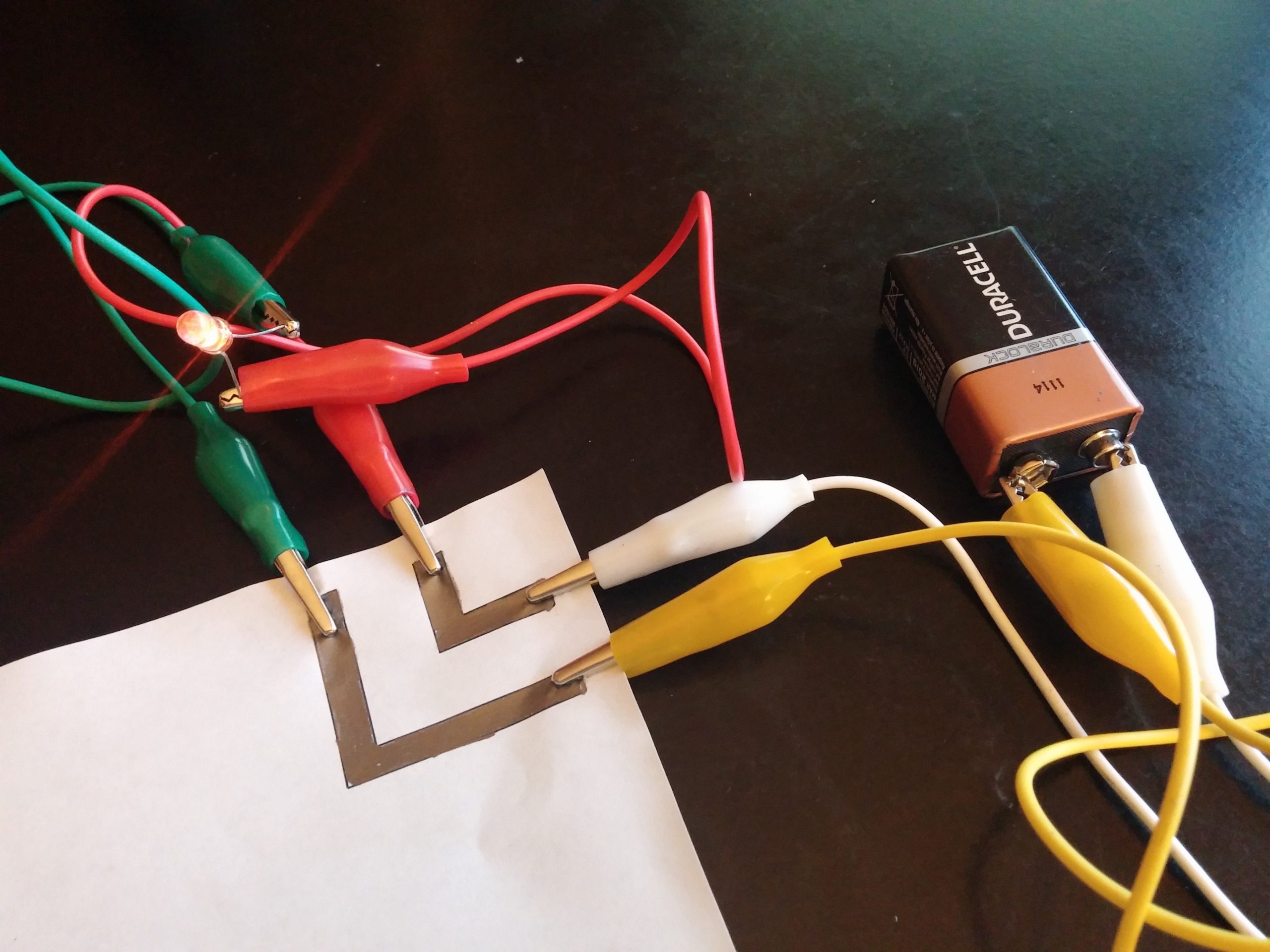
Draw a Circuit: Fun with Graphite
[vc_row][vc_column][vc_column_text css=".vc_custom_1713215921953{padding-bottom: 20px !important;}"]Students explore the conductive properties of graphite and graphene as they create simple circuits.[/vc_column_text][mkd_accordion style="boxed_toggle"][mkd_accordion_tab icon_pack="" title="Question"][vc_column_text]Can thin layers of graphite conduct electricity?[/vc_column_text][/mkd_accordion_tab][mkd_accordion_tab icon_pack="" title="Background"][vc_column_text]What we call “pencil lead” is actually a substance called graphite, which consists of many stacked sheets of carbon atoms. Like a metal, graphite is conductive and therefore can act like a wire on paper to create the circuit. Each sheet of carbon atoms is bonded in honeycomb structure and the single layers are known as graphene (see picture on front page or toy model; also if available, look under microscope at a real graphene flake!). These...